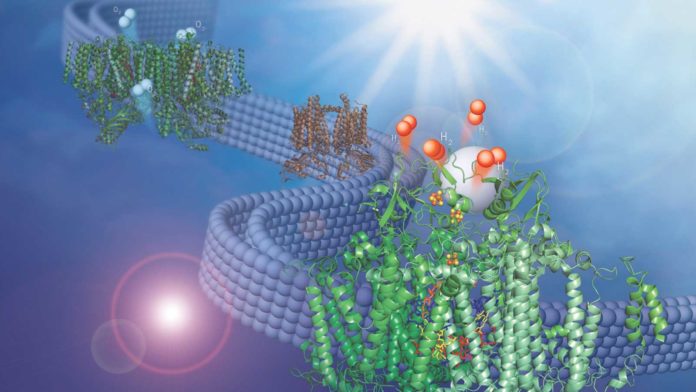
Scientists drove a chemical reaction to plant biology and created a backbone of a convert new process that converts water into hydrogen fuel using energy from the sun. They combined two membrane-bound protein complexes to perform a complete conversion of water molecules to hydrogen and oxygen.
Scientists used a second protein complex called Photosystem II- that uses energy from light to split water and take electrons from it. Using the protein complex, scientists were able to take electrons from water and feed them to Photosystem I.
Lisa Utschig, Argonne chemist said, “The two protein complexes are embedded in thylakoid membranes, like those found inside the oxygen-creating chloroplasts in higher plants. The membrane, which we have taken directly from nature, is essential for pairing the two photosystems. It structurally supports both of them simultaneously and provides a direct pathway for inter-protein electron transfer, but doesn’t impede catalyst binding to Photosystem I.”
“The Z-scheme — which is the technical name for the light-triggered electron transport chain of natural photosynthesis that occurs in the thylakoid membrane — and the synthetic catalyst come together quite elegantly. The beauty of this design is in its simplicity — you can self-assemble the catalyst with the natural membrane to do the chemistry you want.”
“One additional improvement involved the substitution of cobalt or nickel-containing catalysts for the expensive platinum catalyst that had been used in the earlier study. The new cobalt or nickel catalysts could dramatically reduce potential costs.”
“The next step for the research, according to Utschig, involves incorporating the membrane-bound Z-scheme into a living system. Once we have an in vivo system — one in which the process is happening in a living organism — we will really be able to see the rubber hitting the road in terms of hydrogen production.”
The study is published in the journal Chemical Science.