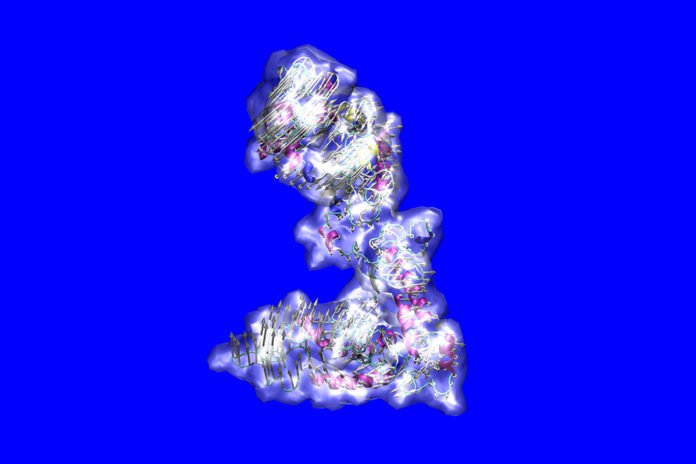
For the first time, MIT specialists have decoded the molecular structure of the complex molecule, tropoelastin, and additionally the details of what can turn out badly with its structure in different hereditarily determined diseases.
Tropoelastin is the precursor molecule of elastin, which along with structures called microfibrils is the key to the flexibility of tissues including skin, lungs, and blood vessels. But the molecule is complex, made up of 698 amino acids in sequence and filled with disordered regions, so unraveling its structure has been a major challenge for science.
Scientists solved this challenge by utilizing a blend of molecular modeling and exploratory perception to fabricate an atom-by-atom picture of the molecule’s structure.
This finding suggests that how certain diverse disease-causing transformations in the single gene that controls the development of tropoelastin change the molecule’s stiffness and dynamic responses, which could at last help in the outline of medicines or countermeasures for these conditions. Other “artificial” transformations incited by the scientists, that don’t relate to any known naturally occurring mutations, can be utilized to better comprehend the capacity of the particular part of the gene influenced by that transformation.
Anna Tarakanova Ph.D. ’17, an MIT postdoc said, “We’re interested in probing a particular region of the molecule to understand the function of that region. In addition to imparting elasticity, the molecule plays a key role in cell signaling and cell adhesion, affecting cellular processes which are driven by interactions with specific sequences within the molecule.”
Scientists also identified the specific changes in the tropoelastin molecule caused by mutations that are associated with known diseases, such as cutis laxa, in which the skin lacks elasticity and hangs loosely. They discovered that a point transformation related to the disease causes changes in the molecule that have suggestions — the component of the sickness really originates from the [changes on the] molecular scale.
Markus Buehler, the Jerry McAfee Professor in Engineering said, “Understanding the structure of this molecule is not only important in the context of disease but can also enable us to translate the knowledge from this biomaterial to synthetic polymers, which can be designed to meet certain engineering needs. Engineering the balance of order and disorder in the context of desired properties could open doors to new designer materials.”
Scientists used a technique that includes molecular dynamics modeling and simulation. The approach consolidates taking a gander at “the global structure of the molecule, to consider the general outline” into which the molecular structure must fit. At that point, they look in detail at local, auxiliary structures inside the molecule, which were separated from a lot of information in the scientific literature from experimental work.
Buehler said, “More generally, I think this approach is applicable to large molecules with a high degree of disorder — and by some estimates, half of the proteins in your body contain regions with a high degree of disorder. This can be a very powerful framework for looking at many kinds of [biological] systems.”
The results appear this week in the Proceedings of the National Academy of Sciences in a paper by Markus Buehler, the Jerry McAfee Professor in Engineering and head of the MIT Department of Civil and Environmental Engineering; Anna Tarakanova PhD ’17, an MIT postdoc; and three others at the University of Sydney and the University of Manchester.
Zsolt Urban, an associate professor of human genetics at the University of Pittsburgh said, “Elastin is necessary for the proper working of stretchy organs such as blood vessels, heart valves, and lungs. However, the full structure of tropoelastin was unknown until now. Scientists now have solved the structure of tropoelastin at the resolution of atoms. This is a remarkable feat given that tropoelastin consists of more than 8,000 atoms.”
The research team also included postdoc Giselle Yeo and professor of biochemistry Anthony Weiss at the University of Sydney, Australia, and professor of biochemistry Clair Baldock at the University of Manchester, in the U.K. The work was supported by the National Institutes of Health, the Office of Naval Research, the National Science Foundation, the Australian Research Council, and the Wellcome Trust.