The researchers from RMIT University, École Polytechnique fédérale de Lausanne (EPFL) and Ludwig Institute for Cancer Research in Lausanne, have devised a new lab-on-a-chip technology that tells scientists how human cells communicate. It offers researchers unprecedented insights into how individual cells behave – something that scientists are discovering is far more complex than previously thought.
Originally, the technology is a sensor through which scientists can isolate single cells analyze them in real time and observe their complex signaling behavior without disturbing their environment.
Distinguished Professor Arnan Mitchell, Director of RMIT’s MicroNano Research Facility, said single cell analysis held great promise for developing new treatments for diseases but a lack of effective analysis technologies was holding back research in the field.
“We know a lot about how groups of cells communicate to fight disease or respond to infections but we still have a lot to learn about individual cells,” Mitchell said.
“Studies have recently shown that you can take two cells of the same type and give them the same treatment but they will respond very differently.
“We don’t know enough about the underlying mechanisms to understand why this happens and we don’t have the right technologies to help scientists figure it out.
“Our solution to this challenge is a complete package – an integrated optofluidic biosensor that can isolate single cells and monitor the chemicals they produce in real-time over at least 12 hours.
“It’s a powerful new tool that will give us a deeper fundamental understanding of cell communication and behavior. These insights will open the way to develop radically new methods for diagnosing and treating disease.”
Seeing how individual cells collaborate and impart is basic to growing new treatments for genuine maladies, to better tackle the energy of the body’s own resistant framework or definitely target defective cells.

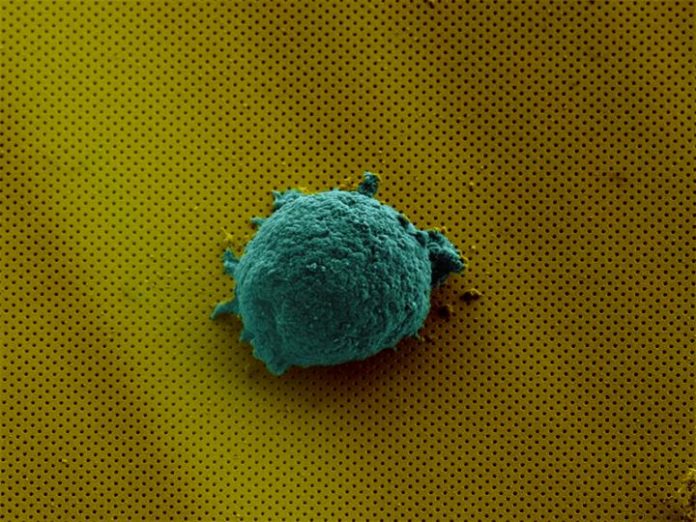
In the study, scientists demonstrate how the technology can be used to examine the secretion of cytokines from single lymphoma cells. Cytokines are small proteins produced by a broad range of cells to communicate to other cells, and they are known to play an important role in responses to infection, immune disorders, inflammation, sepsis, and cancer. The study found the lymphoma cells produced cytokine in different ways, unique to each cell, enabling researchers to determine each cell’s secretion fingerprints.
Mitchell said, “If we can build up a clear picture of this behavior, this would help us sort good cells from bad and enable us to one day develop treatments that precisely target just those bad cells.”
The sensor is the latest adaptation of microfluidic chip which contains tiny channels, pumps, and processors, enabling precise and flexible manipulation of fluids. Basically, microfluidics improves the fluids, what microelectronics improves the situation data – incorporating tremendous amounts of minor handling components into a little chip that is versatile, quick and can be delivered rapidly and proficiently.
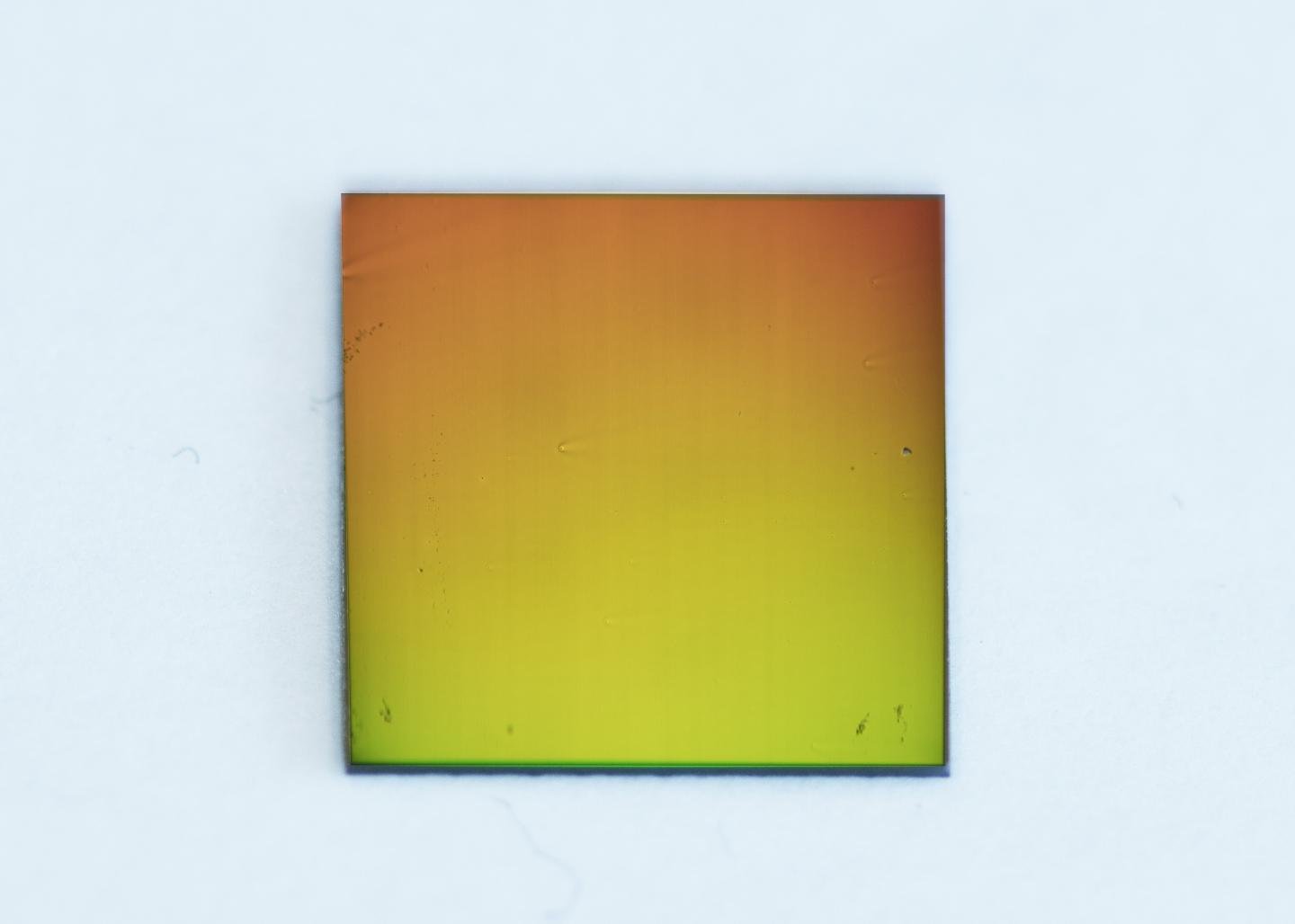
The new practical and scalable innovation is lightweight and versatile, joining microfluidics with nanophotonics. Moreover, it is perfect with conventional magnifying instruments, the biosensor is a thin glass slide covered with a gold film, punctured with billions of tiny nanoholes arranged in a particular example. These nanoholes transmit a single shade of light, because of an optical phenomenon are known as the plasmonic effect.
By observing the color transmitted, researchers can determine the presence of minute quantities of specific chemicals on a slide without any external labels. This detection method enables the continuous monitoring of the chemicals produced from a single cell in real time.
The nanophotonic sensor is coupled to a microfluidic integrated circuit with fluid channels about the size of a human hair. The circuit includes valves to isolate the cell and concentrate its secretions, and systems to regulate the temperature and humidity to sustain the cell.
The work is a collaboration between the laboratory of Bionanophotonic Systems at EPFL, Switzerland, the Integrated Photonics, and Applications Centre in the School of Engineering at RMIT and Ludwig Institute for Cancer Research, Switzerland.
RMIT microfluidic chips have been pivotal in enabling research across a range of areas – from water quality monitoring to the development of point-of-care blood tests for suspected heart attacks that could deliver results while a patient is still in an ambulance.
The paper, “Label-free optofluidic nanobiosensor enables real-time analysis of single-cell cytokine secretion” is published in Small (DOI: 10.1002/smll.201800698).