The aerosol droplets are widely available on the Earth and mimic a vital role in Earth’s chemistry. These droplets are usually generated in the clean air after emissions of gases that nucleate and condense.
Several times organic acids dominate this process. What attracted the scientists most is the unknown mechanism by which organic carboxylic acids dissolve from the gas phase into such aqueous particles.
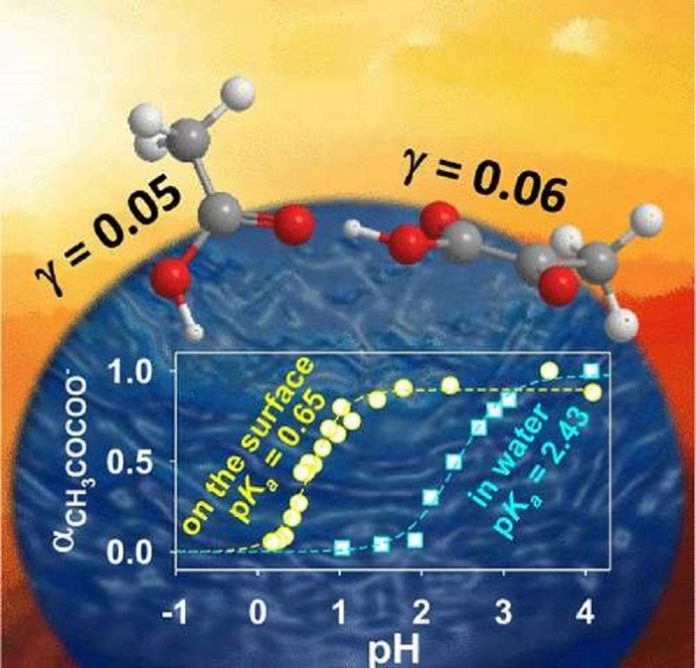
In a new study by the University of Kentucky in collaboration with A.J. Colussi from Caltech. Scientists show that acetic acid and pyruvic acid behave as stronger acids on the air-side on the surface of the water than when they are dissolved in bulk water.
For this reason, the specialists’ utilized online electrospray ionization (OESI) to produce microdroplets, in a procedure that was commanded by pneumatically helped aerosolization of liquid samples at settled pH. The microdroplets in OESI were affected by acid molecules from the gas-stage before the particles were created and distinguished by a mass spectrometer (MS). The strategy empowered the efficient observation of a standout amongst the most fundamental chemical reactions, the exchange of protons from the corrosive particles to the surface of the water.
Marcelo Guzman’s group at the University of Kentucky said, “They were presumably more prone to transfer a proton as they reached the surface of water coming from the air, even more than 50 and 500 times.”
The researchers concluded that the behavior and stability of each acid and base pair at the interface played an important role in this phenomena. The results of this work will help to interpret how common carboxylic acids found in aerosols, clouds and fog waters behave in the atmosphere and contribute to design strategies for reducing air pollution.
The co-authors of the study include J. Eugene, Elizabeth A. Pillar, Agustín J. Colussi, and Marcelo I. Guzman. Langmuir. The paper is published in the Langmuir.