Multimetallic clusters- ordinarily made out of at least three metals – are earning consideration as they display properties that can’t be accomplished by single-metal materials. On the off chance that an assortment of metal components are freely blended, it is normal that up ’til now obscure substances are found and highly-functional materials are created.
Up until this point, nobody had revealed the multimetallic clusters blended with in excess of four metal components so far as a result of a troublesome division of various metals. One plan to beat this trouble is scaling down of cluster sizes to one-nanometer scale, which forces the diverse metals to be mixed in a little space. Be that as it may, there was no real way to understand this thought.
A team of scientists, including Takamasa Tsukamoto, Takane Imaoka, Kimihisa Yamamoto and associates, has built up an atom hybridization technique, which has understood the first-since forever union of multimetallic clusters comprising of in excess of five metal components with exact control of size and composition.
Researchers in Japan have found a way to create innovative materials by blending metals with precision control. Their approach, based on a concept called atom hybridization.

CREDIT
Takamasa Tsukamoto
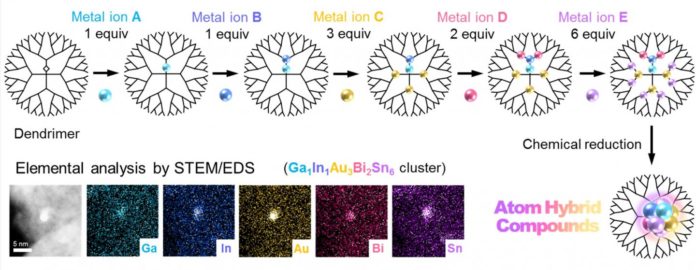
This method employs a dendrimer template that serves as a tiny “scaffold” to enable controlled accumulation of metal salts. After precise uptake of the different metals into the dendrimer, multimetallic clusters are obtained by chemical reduction.
Interestingly, a customary strategy without the dendrimer yields enlargement of cluster sizes and partition of various metals. The group effectively exhibited the arrangement of five-element clusters made out of gallium (Ga), indium (In), gold (Au), bismuth (Bi) and tin (Sn), and additionally press (Fe), palladium (Pd), rhodium (Rh), antimony (Sb) and copper (Cu), and a six-element cluster comprising of Ga, In, Au, Bi, Sn and platinum (Pt). Moreover, they indicate the likelihood of making clusters made out of eight metals or more.
This atom hybridization technique utilizing the dendrimer format can incorporate ultrasmall multimetallic clusters with exact control of size and composition. There are in excess of 90 metals in the periodic table. With infinite combinations of metal components, atomicity, and composition, this technique will open up another field of science on a one-nanometer scale. The present investigation denotes a noteworthy advance forward in making, for example, yet-unknown creative materials.
The study is published in the journal Nature Communications.