Hemoglobin (Hb or Hgb) is a protein in red blood cells that carries oxygen throughout the body. But it succeeds only when it forms a complex. The same applies to chlorophyll.
In order to understand the function of such complex molecules, it’s worth investigating similar but simpler structures in the lab. Now, scientists at the Vienna University of Technology and research groups from Trieste, phthalocyanines came up together and studied whose sub-atomic ring structure closely takes after the vital areas of hemoglobin or chlorophyll. It worked out that the center of these ring structures can be exchanged into various states with the assistance of green light, which changes their chemical behavior.
According to scientists, the study will help them understand biological processes. It could also pave the way for new possibilities for using the tricks of nature in the laboratory for other purposes.
Prof. Günther Rupprechter from the Institute of Material Chemistry at the Vienna University of Technology said, “The phthalocyanines that we study are dyes with a characteristic ring structure. Crucial to this ring structure is that it can pick up an iron atom in its center – just like the Häme, the ring-shaped red dyes in hemoglobin. Chlorophyll, in turn, has a similar ring that holds on to magnesium.”
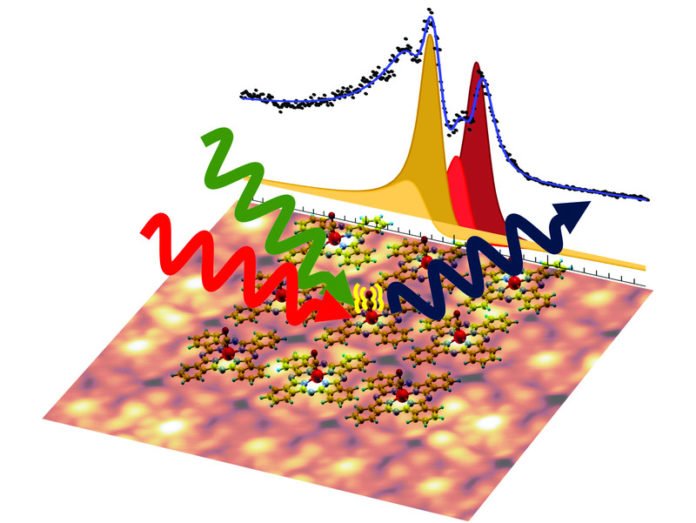
Matteo Roiaz, who conducted the experiments together with Christoph Rameshan said, “In contrast to the more complicated natural models, the phthalocyanine dyes can be regularly placed side by side on a surface, such as tiles on the bathroom wall. The rings were regularly applied to a graphene layer, so a two-dimensional crystal of all the same dye rings was created. This has the advantage that we can examine many molecules at the same time and thus obtain much clearer measurement signals.”
Carbon monoxide particles filled in as tests for the examination of the rings: they can append to the iron atom which is held in the center of the ring. From the vibration of the carbon monoxide particle, one can pick up data about the condition of the iron atom.
In order to study the vibration, scientists irritated the molecule using laser light. This brought about an outcome that appeared to be counterproductive at first look.
Günther Rupprechter said, “It was not simply a frequency of vibration of carbon monoxide measured, but four different – no one had expected. The iron atoms are all identical, so the CO molecules attached to them should all show the same behavior.”
“As it turned out, the green light portion of the laser was responsible for a remarkable effect: in fact, all the iron atoms were originally identical, but the interaction with a green light can switch them to different states. That this also changes the oscillation frequency of the CO molecule on the iron atom shows us how sensitively such structures react to tiny changes.”
“That’s why the biomolecules in our bodies have such a complex structure: the widely branched protein branches have a minimal impact on the states of the metal atom – but this minimal impact has very important implications.”
“Until now, similar effects could only be studied at extremely low temperatures and in ultrahigh vacuum. In the laboratory, we even have two measuring methods in which such biologically relevant phenomena can be measured at room temperature and atmospheric pressure, with and without a green light.”
“This opens up new possibilities for a better understanding of the chemical behavior of biological substances; it could also offer the opportunity to tailor novel molecules in order to optimize them for nature-specific chemical purposes on the example of nature.”
The study is published in the journal Nature Communications.