MIT scientists have designed a new peptide that can upset a key protein that many sorts of tumors, including a few types of lymphoma, leukemia, and bosom disease, need to survive.
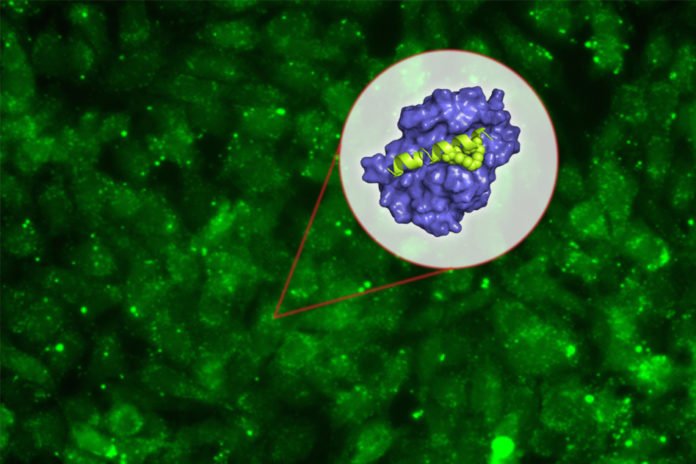
The new peptide focuses on a protein called Mcl-1, which enables tumor cells to stay away from the phone suicide that is generally instigated by DNA harm. By blocking Mcl-1, the peptide can compel tumor cells to experience modified cell demise.
Some tumor cells are extremely subject to Mcl-1, which is the last line of resistance shielding the cell from biting the dust. It’s an exceptionally alluring target.
Mcl-1 has a place with a group of five proteins that assume parts in controlling customized cell demise, or apoptosis. Each of these proteins has been observed to be overactive in various sorts of disease. These proteins shape what is called an “apoptotic barricade,” implying that phones can’t experience apoptosis, notwithstanding when they encounter DNA harm that would typically trigger cell passing. This enables growth cells to survive and multiply unchecked and has all the earmarks of being a critical way that cells wind up noticeably impervious to chemotherapy tranquilizes that harm DNA.
Disease cells have numerous procedures to remain alive, and Mcl-1 is an imperative factor for a ton of intense myeloid leukemias and lymphomas and some strong tissue growths like bosom tumors. Articulation of Mcl-1 is upregulated in numerous diseases, and it supposedly was upregulated as a protection factor to chemotherapies.
Numerous pharmaceutical organizations have endeavored to create drugs that objective Mcl-1, yet this has been troublesome in light of the fact that the communication between Mcl-1 and its objective protein happens in a long extension of 20 to 25 amino acids, which is hard to obstruct with the little particles commonly utilized as medications.
Peptide drugs, then again, can be intended to tie firmly with Mcl-1, keeping it from interfacing with its characteristic restricting accomplice in the cell. Keating’s lab spent numerous years outlining peptides that would tie to the segment of Mcl-1 engaged with this communication — yet not too different individuals from the protein family.
When they thought of some encouraging hopefuls, they experienced another obstruction, which is the trouble of motivating peptides to enter cells.
Amy Keating, an MIT professor of biology said, “We were exploring ways of developing peptides that bind selectively, and we were very successful at that, but then we confronted the problem that our short, 23-residue peptides are not promising therapeutic candidates primarily because they cannot get into cells.”
To overcome this, scientists then collaborated with Walensky’s lab, which had previously shown that “stapling” these small peptides can make them more stable and help them get into cells. These staples, which consist of hydrocarbons that form crosslinks within the peptides, can induce normally floppy proteins to assume a more stable helical structure.
They made around 40 variations of their Mcl-1-blocking peptides, with staples in various positions. By testing these, they recognized one area in the peptide were putting a staple not just enhances the particle’s dependability and causes it get into cells, yet in addition influences it to tie significantly more firmly to Mcl-1.
Keating said, “The original goal of the staple was to get the peptide into the cell, but it turns out the staple can also enhance the binding and enhance the specificity. We weren’t expecting that.”
The analysts tried their best two Mcl-1 inhibitors in growth cells that are subject to Mcl-1 for survival. They found that the inhibitors could murder these malignancy cells all alone, with no extra medications. They additionally found that the Mcl-1 inhibitors were exceptionally particular and did not execute cells that depend on different individuals from the protein family.
Keating says that all the more testing is expected to decide how successful the medications may be in fighting particular malignancies, regardless of whether the medications would be best in the mix with others or all alone, and whether they ought to be utilized as first-line drugs or when growths end up noticeably impervious to different medications.
Kritzer, who was not involved in the research said, “There have been a lot of biologists and biochemists studying essential interactions of proteins, with the justification that with more understanding of them, we would be able to develop drugs that inhibit them. This work now shows a direct line between a biochemical and biophysical understanding of protein interactions to an inhibitor.”