Scientists at the Berkeley Lab have developed a library of artificial proteins that offers a computerized, high-throughput technique for correctly designing new peptoids—protein-like polymers with a precise sequence of monomer units—that chelate lanthanides, for example, gadolinium, a typical fixing in MRI contrast agents, and actinides, for example, plutonium.
What’s more, these proteins can proficiently bound to lanthanides and actinides, heavy metals that make up the so-called f-block elements at the bottom of the periodic table. Scientists incorporated specially designed monomers with f-block-binding properties onto peptoid scaffolds.
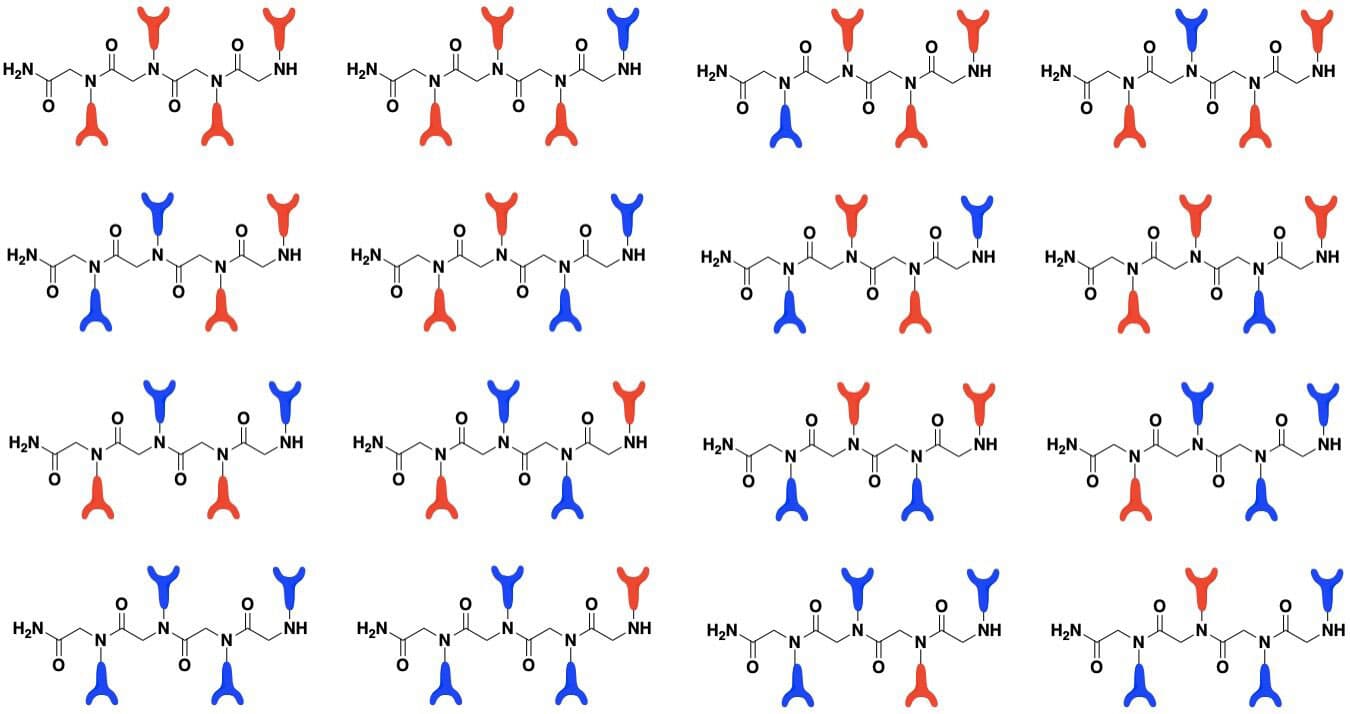
For this study, scientists assembled two bio-inspired hydroxypyridinone and catecholamide monomers onto molecular complexes called “tetramers,” yielding a library of 16 chelating peptoids (also known as “ligands”). They then used a luminescence-based technique to measure how well each chelating peptoid coordinated to the lanthanide cations (positively charged ions) europium and terbium.
They found that the chelating systems, including three and four hydroxypyridinone functional groups, show a high affinity for lanthanide metals, and in particular, europium.
Meanwhile, these peptoid-based chelators could be used to design ligands tailor-made for a wide range of applications with f-block metals, such as chemical separation processes, optical device optimization, and pharmaceutical development. Moreover, if scientists extend the technique to incorporate additional monomers, it could lead to much larger libraries.
The study was led by Rebecca Abergel, a faculty scientist in Berkeley Lab’s Chemical Sciences Division and assistant professor in UC Berkeley’s Nuclear Engineering Department.
The study is reported in the journal Chemical Science.